Lateral flow assay for respiratory viruses
CRISPR/Cas9-based point-of-care diagnosis for SARS-CoV-2
Applications
- Point-of-care diagnostics for viral respiratory pathogens
- Rapid detection of SARS-CoV-2
Key Benefits & Differentiators
- Rapid and multiplexable: Detection and differentiation of SARS-CoV-2, influenza A and B, and/or respiratory syncytial virus (RSV) in a single reaction
- Low cost: the assay doesn’t require specialized training, reagents or equipment
Technology Summary
The COVID-19 pandemic caused by coronavirus-2 has led to significant disruptions worldwide with over 200 million people infected and over 4 million deaths. Testing for exposure and infection to coronavirus is important in addressing its spread and informs the ability of public health professionals in the deployment of containment measures including contact tracing and quarantining. Despite this importance, widespread testing remains a significant hurdle. Currently, COVID-19 testing requires labor-intensive RNA isolation, specialized instrumentation for real time RT-PCR, and results take 1 to 2 days to obtain. Thus, there is a critical need for a rapid, high throughput COVID-19 point-of-care test. In addition, there are two types of COVID, (Type I and Type II) and because of differences in translational efficiencies, Type II virus particles are believed to be more transmissible than Type I virus particles. Thus, there is a need for a simple test to identify and differentiate between the two types.
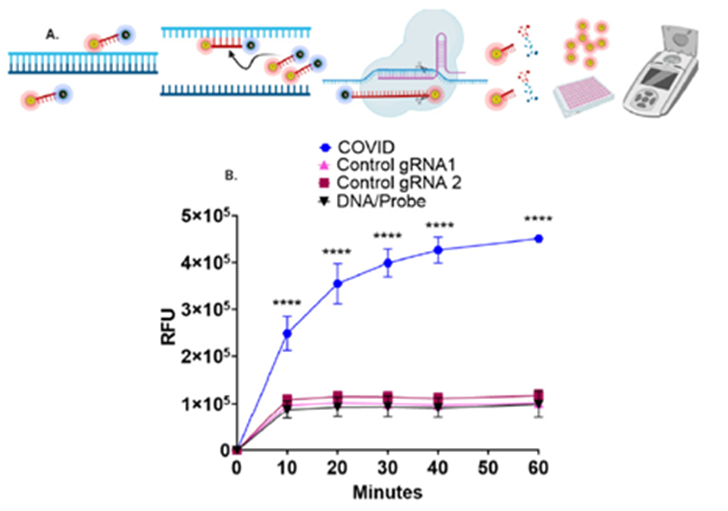
Researchers at the University of Minnesota have developed a sensitive assay for the detection of coronavirus and disambiguation between Type I and II viruses. Briefly researchers used commercially available reagents to develop a CRISPR/Cas9-based lateral flow assay (LFA) that can detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequences with single base specificity. They also developed a rapid, multiplex fluorescence CRISPR/Cas9 nuclease cleavage assay capable of detecting and differentiating SARS CoV-2, influenza A and B, and respiratory syncytial virus in a single reaction. This method and assay require minimal equipment and represent a simplified platform for field-based deployment. Overall, the LFA procedure promotes field-based diagnostic capabilities with low cost and little need for special infrastructure.
Phase of Development
TRL: 4-6Laboratory proof of concept tested.
Desired Partnerships
This technology is now available for:- License
- Sponsored research
- Co-development
Please contact our office to share your business’ needs and learn more.
Researchers
- Mark J. Osborn, PhD Assistant Professor, Department of Pediatrics
-
expand_more library_books References (1)
- Osborn, M.J., Bhardwaj, A., Bingea, S.P., Knipping, F., Feser, C.J., Lees, C.J., Collins, D.P., Steer, C.J., Blazar, B.R. and Tolar, J. , CRISPR/Cas9-Based Lateral Flow and Fluorescence Diagnostics
-
expand_more cloud_download Supporting documents (1)Product brochureLateral flow assay for respiratory viruses.pdf