First-in-class drug lead candidate (A229) for treating ocular diseases
PPARα agonist, A229, exhibits strong potency and demonstrates efficacy in in-vitro models of diabetic retinopathy.
Applications
- Ocular disease treatment
- Research tool
Key Benefits & Differentiators
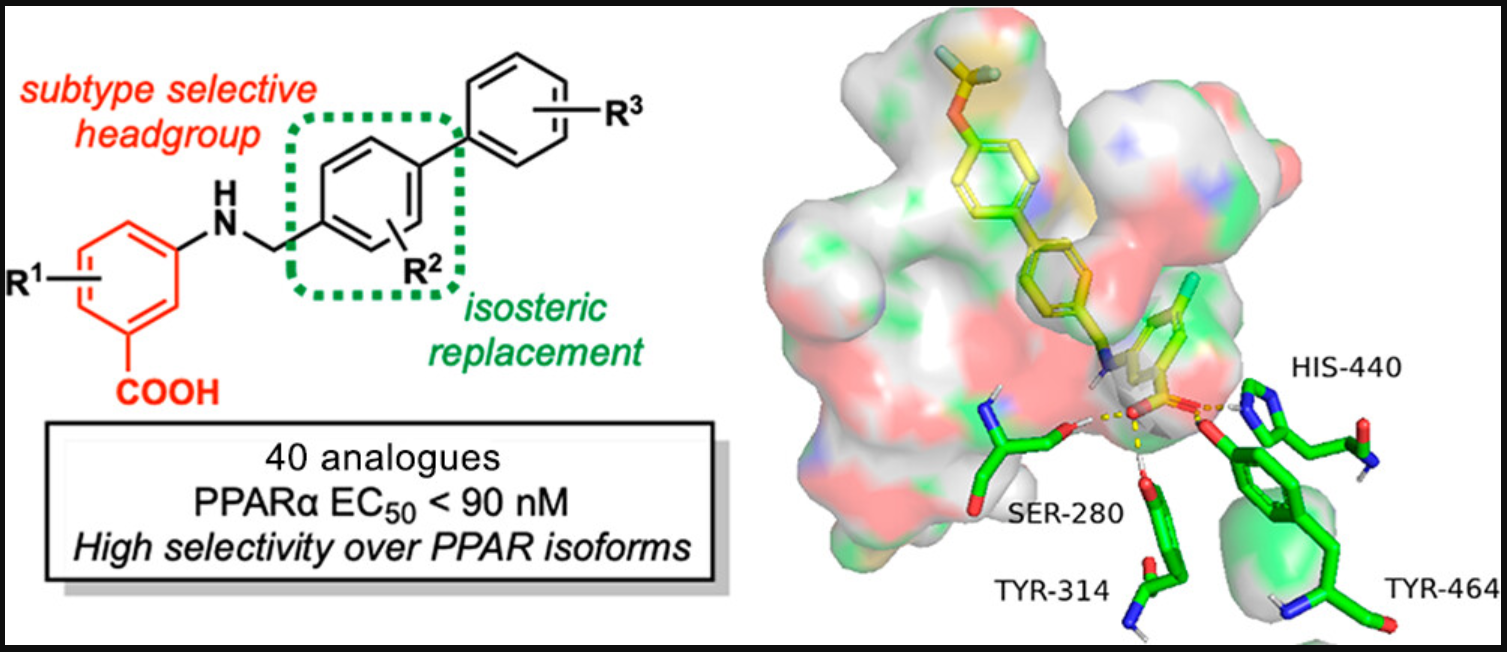
- Subtype selectivity: A229 shows subtype selectivity for PPARα over other isoforms, minimizing potential off-target effects.
- Enhanced potency: A229 exhibits potent activity, potentially reducing the required dosage and side effects while maintaining therapeutic efficacy.
- Efficacy in retinal cells: In hRPE cells under conditions that model DR, A229 upregulates PPARα target genes, improves cell viability, reduces VEGF production, and protects against ROS production.
Technology Overview
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) stand as leading causes of blindness and low vision in the United States. The conventional treatment for these conditions involves direct intraocular injection of anti-VEGF antibodies. However, this treatment method presents challenges, including the need for recurrent injections and a significant percentage (∼40%) of patients not responding adequately to therapy. Consequently, there is a pressing need for novel therapies that surpass or complement current approaches. Such innovations would greatly benefit patients, particularly those who remain refractory to anti-VEGF treatment options.
Researchers from the University of Minnesota have developed a first-in-class drug lead candidate, A229, for potential treatment of ocular diseases. A229 targets Peroxisome proliferator-activated receptor α (PPARα), a ligand-activated transcription factor that is involved in lipid metabolism of various tissues. Previous studies have shown that PPARα agonism significantly reduces the progression of DR, but currently available drugs that target PPARα suffer from low affinity and lack selectivity among PPAR subtypes. A229 exhibits good potency and subtype selectivity for PPARα when assessed in a commercial primary luciferase-based cell assay. In human retinal pigment epithelial (hRPE) cells under conditions that model DR, A229 upregulates PPARα target genes, improves cell viability, reduces VEGF production, and protects against ROS production, aligning with PPARα agonism expectations in a disease-relevant context. Based on this data, A229 is a promising drug candidate for further advanced in-vitro and in-vivo model characterization, with potential for commercial development as a treatment for ocular diseases.
Phase of Development
TRL: 3-4In-vitro data has been generated for A229. In vivo studies are underway.
Desired Partnerships
This technology is now available for:- License
- Sponsored research
- Co-development
Please contact our office to share your business’ needs and learn more.
Researchers
- Adam Duerfeldt, PhD Associate Professor, Department of Medicinal Chemistry
-
expand_more library_books References (1)
- Julia J. Lee, Ziwei Hu, Yuhong Anna Wang, Dinesh Nath, Wentao Liang, Yi Cui, Jian-Xing Ma, and Adam S. Duerfeldt (2023), Design, Synthesis, and Structure–Activity Relationships of Biaryl Anilines as Subtype-Selective PPAR-alpha Agonists, ACS Med. Chem. Lett., 14, 6, 766–776
-
expand_more cloud_download Supporting documents (1)Product brochureFirst-in-class drug lead candidate (A229) for treating ocular diseases.pdf