Nanorings for cell-mediated drug delivery
Farnesylated chemically self-assembled nanorings that facilitate targeted drug delivery between cells.
Applications
- Drug delivery
- Monitoring cellular communication
- Research tool
Key Benefits & Differentiators
- Improved Targeting: f-CSANs enable precise tissue targeting, boosting drug concentration at the target site and enhancing treatment efficacy.
- Low Immunogenicity: f-CSANs reduces immunogenicity by mimicking natural trogocytosis processes.
- Versatility: CSAN-assisted cell-cell cargo transfer (C4T) process is receptor-specific and relies on direct cell-cell interactions, making it a versatile platform for delivering various bioactive species.
Technology Overview
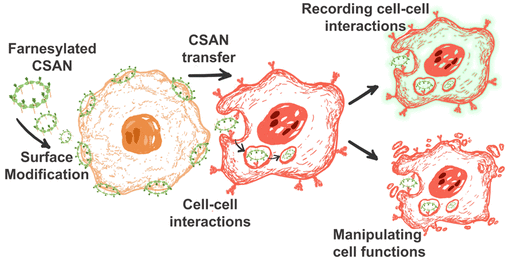
Anti-cancer agents often show suboptimal performance in vivo due to issues like poor tumor targeting, low solubility, and rapid clearance. Drug delivery systems have been developed to overcome these challenges by increasing drug concentration at the target site. Nano- and micro-based nanocarriers, including nanoparticles, bacteria, and viruses, are commonly used to improve drug stability and transport. However, nanocarriers face limitations like poor blood-brain barrier penetration and liver clearance. Cell-mediated drug delivery systems, using cells like red blood cells, neutrophils, macrophages, and stem cells as carriers, have emerged as a promising solution. They offer prolonged circulation, specific tissue targeting, and low immunogenicity, enhancing therapeutic outcomes while minimizing side effects.
Researchers at the University of Minnesota have developed farnesylated chemically self-assembled nanorings (f-CSANs). These biomimetic trogocytosis vehicles facilitate directional cargo transfer between cells, enabling efficient communication by delivering bioactive species. By stably modifying the membranes of sender cells with targeted f-CSANs, cargo can be efficiently transferred to receiver cells expressing the appropriate receptors via endocytosis. This innovative CSAN-assisted cell-cell cargo transfer (C4T) process has been demonstrated to be receptor-specific, relying on direct cell-cell interactions, the rate of receptor internalization, and the level of receptor expression. Notably, C4T facilitates the delivery of apoptosis-inducing drugs and antisense oligonucleotides between cells. This versatile biomimetic trogocytosis platform holds significant promise for advancing cell-based drug delivery strategies.
Phase of Development
TRL: 3-4CSAN-assisted cell-cell cargo transfer has been efficiently demonstrated in vitro.
Desired Partnerships
This technology is now available for:- License
- Sponsored research
- Co-development
Please contact our office to share your business’ needs and learn more.
Researchers
- Carston R. Wagner, PhD Distinguished Teaching Professor & Department Head, Department of Medicinal Chemistry
- Mark Distefano, PhD Distinguished McKnight Professor & Merck Professor, Department of Chemistry
-
expand_more library_books References (1)
- Yiao Wang, Lakmal Rozumalski, Ozgun Kilic, Caitlin Lichtenfels, Jacob Petersberg, Mark D. Distefano, and Carston R. Wagner , Engineering Biomimetic Trogocytosis with Farnesylated Chemically Self-Assembled Nanorings, Biomacromolecules
-
expand_more cloud_download Supporting documents (1)Product brochureNanorings for cell-mediated drug delivery.pdf