MNmask Emergency-Use Face Masks
A design package for three styles of emergency-use face masks.

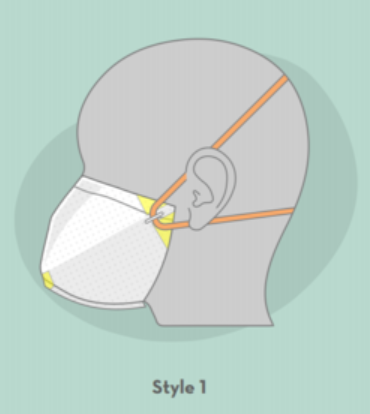


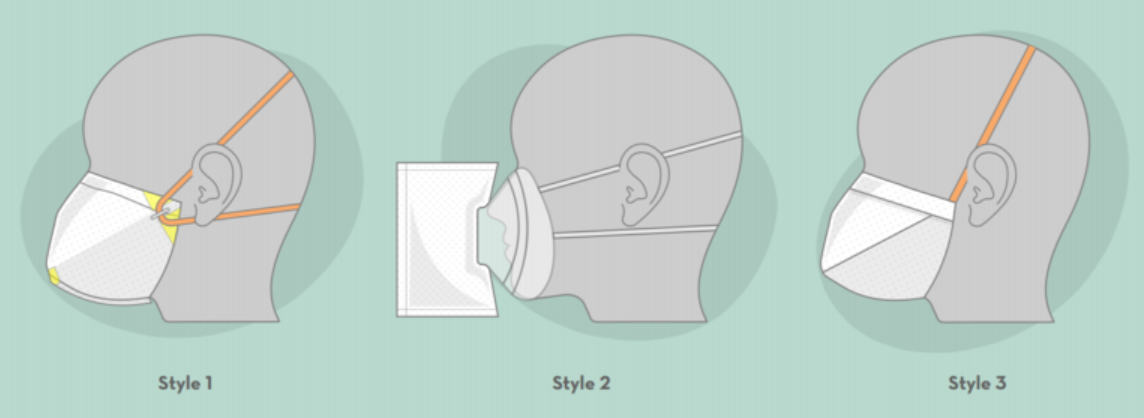
Applications
Face masks for use only when other FDA cleared products are not available.
Key Benefits & Differentiators
- Does not require complex and expensive manufacturing processes or skilled labor
- Uses commercially available industrial grade air filter and widely-available components
- Easy, scalable manufacturing process
MNmask - Overview
To address the growing shortage of protective masks, researchers at the University of Minnesota have designed, prototyped, and tested the MNmasks - face masks that could be produced easily and potentially used when safer alternatives such as N95s are not available. MNmasks use commercially available filter media, components sourced from non-endangered supply chains, and a straight-forward, easy fabrication process that does not require specialized equipment or a highly skilled workforce. Three different styles of MNmasks have been designed and tested:
- Style 1: A face mask featuring heat-sealed contour, foam for comfort and fit, and versatile band placement for a complete seal.
- Style 2: A filter accessory for a reusable anesthesia face mask.
- Style 3: A general-purpose face mask that can be built using only scissors, ruler and a stapler.
These masks use commercially available air filter media, which is typically used in industrial applications. Other necessary components include commercially available tape, rubber bands, staples, and adhesive-backed foam and bendable fixtures. The fabrication process starts with a single rectangular sheet of filter media, and is hand-assembled by heat sealing, folding, stapling and taping. Style 3 requires only a ruler and an office stapler to hand-assemble.
The MNmask project was made possible through support and funding from the University of Minnesota Institute for Engineering in Medicine.
Phase of Development
- Qualitative and quantitative testing of all three styles has occurred with the test protocols and results described in the design package. Thousands of MNmasks have been produced.
- Design files are ready for distribution.
Ready for Licensing
The design files of this device are now available for download at no cost upon execution of the license (provided on the right column of this page). Please contact mnmask@umn.edu for technical inquiries.
Disclaimers
No representations or warranties are being made about these face masks and users assume all risk in face mask use.
(1) These face masks body contact materials are: non-woven polyester, vinyl foam strip, rubber bands (some are non-latex and others contain latex), pressure sensitive acrylic adhesive (could possibly touch face), duct tape, galvanized steel staples, and skin tape (if needed for additional seal). These face masks are intended for single use only.
(2) These face masks have not been FDA cleared or approved; Use only when other FDA cleared products are not available. These products have been authorized by FDA under an EUA for use by health care personnel as personal protective equipment to help prevent the spread of infection or illness in healthcare settings and by the general public to help slow the spread of the virus during the COVID19 pandemic.
(3) These face masks are authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of products under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1) (the “Emergency Declaration”), unless the authorization is terminated or revoked sooner.
(4) Holders of these face masks must dispose of the face masks once the Emergency Declaration terminates or is revoked.
(5) Avoid use of these face masks in any surgical setting or (i) where significant exposure to liquid, bodily or other hazardous fluids, may be expected; (ii) in a clinical setting where the infection risk level through inhalation exposure is high; (iii) in the presence of a high intensity heat source or flammable gas. These face masks are not intended for antimicrobial, antiviral, or infection protection.
(6) These face masks should NOT be used as a replacement for conventional and approved Personal Protective Equipment (“PPE”). These face masks have not been industry tested nor have they been NIOSH approved.
Researchers
-
expand_more cloud_download Supporting documents (1)Product brochureMNmask Emergency-Use Face Masks.pdfAdditional files may be available once you've completed the transaction for this product. If you've already done so, please log into your account and visit My account / Downloads section to view them.